After years of theoretical work, scientists from Eötvös Loránd University, Dr. Ádám Sturm and Dr. Tibor Vellai, prove their aging theory experimentally in a major breakthrough published in Nature Communications.
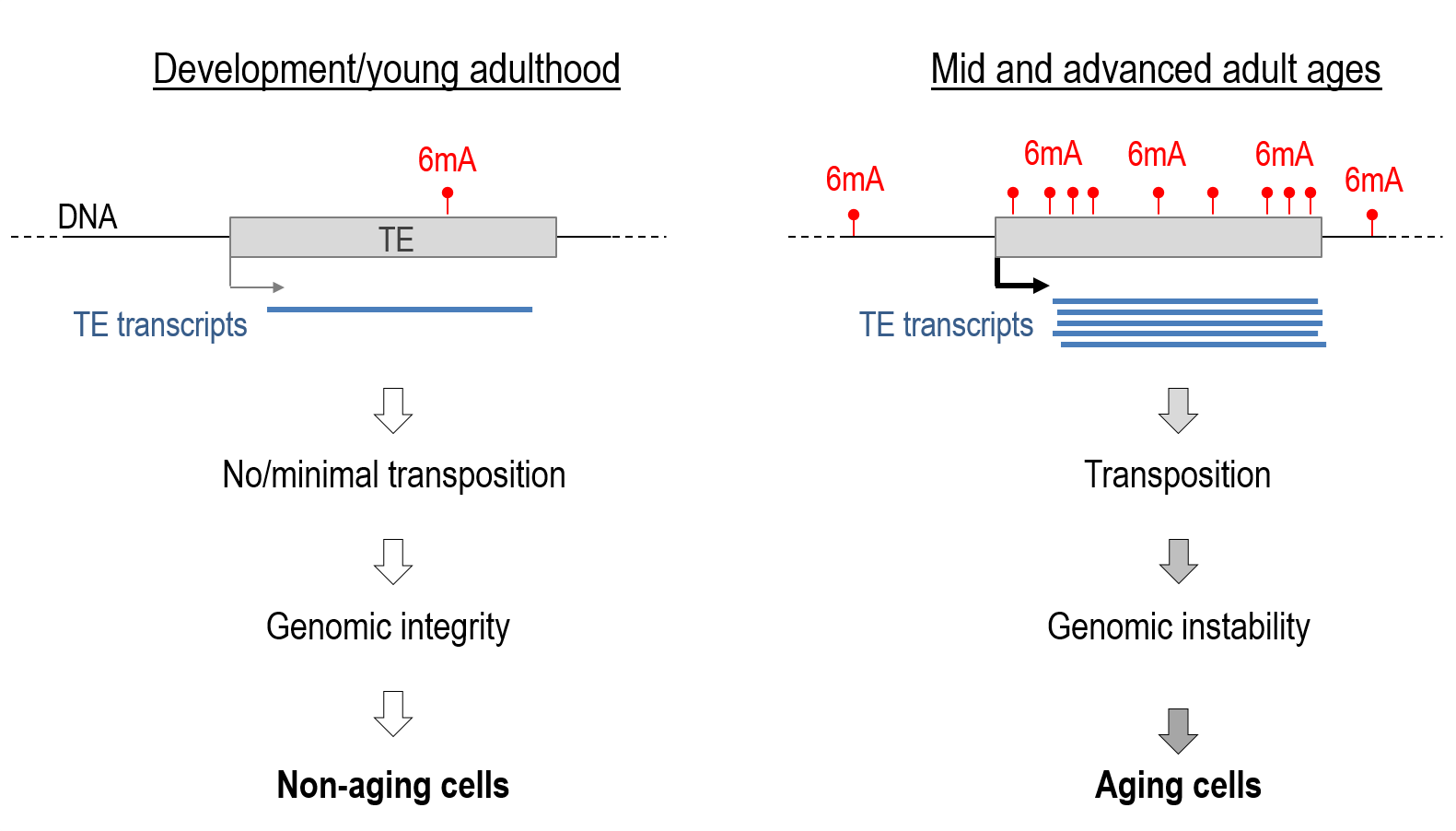
“Jumping genes”, also known as transposable elements (TE), account for more than half of our DNA. As we age, these jumping genes become more active because of an epigenetic DNA change called ‘adenine methylation’ or ‘6mA’. When these genes move and insert themselves into new locations within the DNA, they can interfere with the operation of other genes or important regions. This can change how those genes work or even stop them from working altogether, affecting the overall function and health of the cell. Credit: Sturm, Á., et al., 2023
Pioneering Hungarian aging researchers Dr. Ádám Sturm and Dr. Tibor Vellai have taken a monumental leap in the understanding of aging and, potentially, biological immortality. Their latest publication in Nature Communications comes as the culmination of years of theoretical and experimental works, suggesting the pivotal role of transposable elements (TEs) in aging and the Piwi-piRNA pathway inhibiting these elements in the non-aging biological systems.
The Piwi-piRNA pathway, has previously been proposed by the duo as the hidden mechanism behind the non-aging feature of the germline, cancer stem cells, and notably, the enigmatic Turritopsis dohrnii, commonly known as the “immortal jellyfish.”
In previous landmark articles entitled “The mechanism of aging: primary role of transposable elements in genome disintegration” (2015) and “The Piwi-piRNA pathway: road to immortality” (2017), Dr. Sturm and Dr. Vellai theorized the profound relationship between the Piwi-piRNA system and intriguing concept of biological immortality.
“Through years of dedicated research, we’ve long hypothesized the connection between the Piwi-piRNA pathway and non-aging biological systems. Today, we’ve experimentally proven its role in extending lifespan in a multicellular organism,” said Dr. Ádám Sturm commenting on the experiment in which an element of the Piwi-piRNA system from the non-aging germline was inserted and overexpressed in aging somatic cells. It was discovered that this intervention significantly increases the animal’s lifespan.
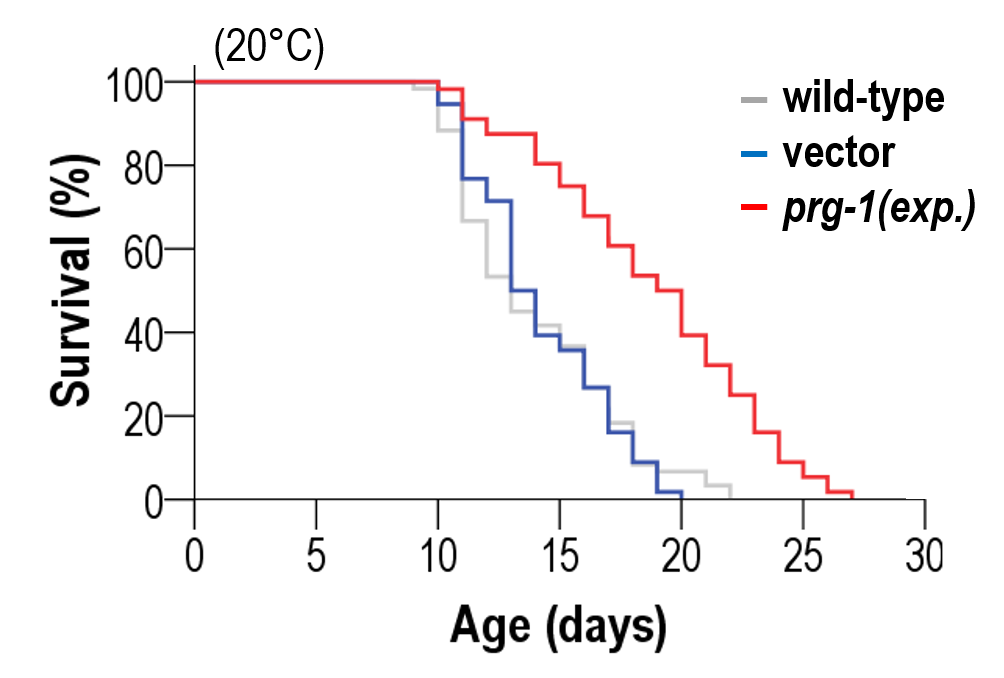
In C. elegans, somatic expression of prg-1 (Piwi-related gene)/Piwi, which is normally active in the non-aging germline only, significantly extends lifespan. Credit: Sturm, Á., et al., 2023
Dr. Tibor Vellai commented, “Our findings bridge the gap between theory and empirical evidence, ushering in a new era of genetic exploration. By harnessing the power of this pathway, the possibilities for extending life and enhancing healthspan are monumental.”
The new paper entitled “Downregulation of transposable elements extends lifespan in Caenorhabditis elegans” focuses on the mobility of TEs and their role in causing genomic instability. The research team used the nematode Caenorhabditis elegans as a model organism to explore this relationship.
“We’ve always been intrigued by the fundamental problem of whether the increasing mobilization of TEs is a cause or a consequence of aging,” said Dr. Sturm. “With this study, we’ve made a pivotal discovery: downregulating active TE families indeed extends lifespan. This indicates that TE mobilization drives the aging process.”
Dr. Vellai added, “It’s a significant step forward. The role of TEs in aging hasn’t been this comprehensively understood before. The results showcase that TEs are a crucial genetic determinant of lifespan.”
For those unfamiliar, TEs are segments of DNA that can change their positions within the genome, possibly leading to mutations that affect the host organism’s health and longevity. The Piwi-piRNA pathway effectively suppresses TEs in the non-aging germline (cells that are set aside during early development and later become eggs or sperm). However, as somatic cells age and lose the pathway’s effects, TEs become gradually mobile, causing genomic instability.
The team not only demonstrated that the expression of TEs is related to aging using a research-specific total RNA sequencing method, but they also extensively employed RNA interference-mediated downregulation to study specific active TE gene families, such as Tc1 and Tc3, in worms. They discovered that silencing TEs slows down the aging process. They also revealed that if several active TE families were silenced at the same time, the effect was additive, thus further proving that extreme longevity can be achieved if all active TEs are silenced simultaneously, as in the case of non-aging biological systems.
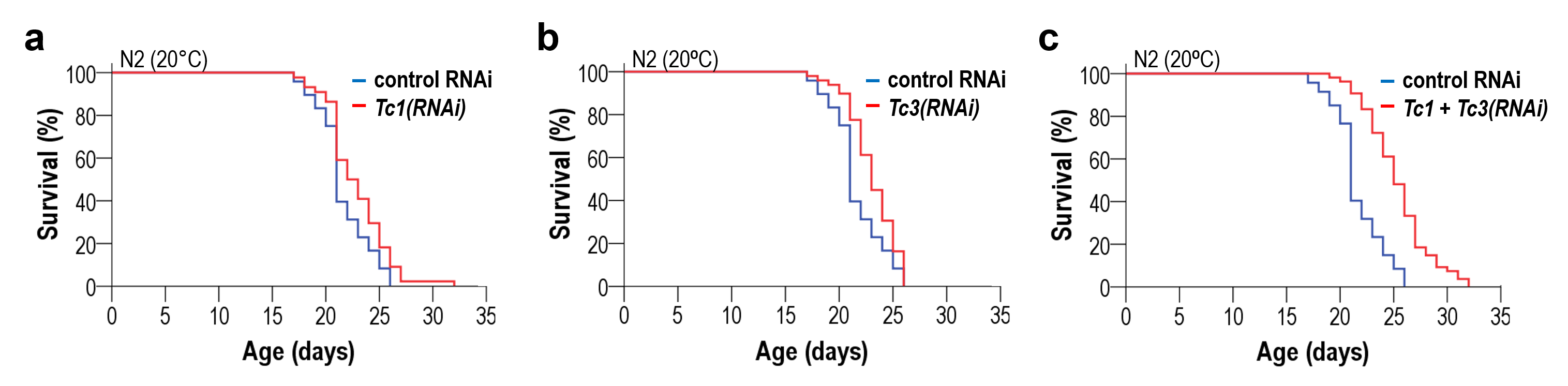
Downregulation of active transposable element (TE) families extends lifespan in C. elegans. Inhibition of Tc1 (a), Tc3 (b) and both TE families (c) promotes longevity. Simultaneous downregulation of Tc1 and Tc3 displays an additive effect. Credit: Sturm, Á., et al., 2023
“In our lifespan assays, by merely downregulating TEs or somatically overexpressing the Piwi-piRNA pathway elements, we observed a statistically significant lifespan advantage,” Dr. Vellai explained. “This opens the door to a myriad of potential applications in the world of medicine and biology.”
Furthermore, the research introduced the idea that DNA N6-adenine methylation, which is an epigenetic modification, rises with age at TE stretches specifically. This modification was observed using Single-molecule real-time (SMRT) sequencing and a newly developed PCR-based method for detecting N6-adenine methylation, which indicated an increase in their transcription as the animal aged.
Dr. Sturm emphasized the potential implications of this discovery. “This epigenetic modification may pave the way for a method to determine age from DNA, providing an accurate biological clock.”
The study provides not just a glimpse into the intricate mechanisms of aging but also offers avenues for further research into how controlling TE activity might affect overall health and longevity.
Both Dr. Sturm and Dr. Vellai urged researchers worldwide to delve deeper into this discovery, believing it to be a starting point for further revelations about aging. “This is just the beginning,” said Dr. Vellai, “the path to understanding and, perhaps, controlling aging and all diseases of old age is clearer than ever.”
More information: Sturm, Á., et al. Downregulation of transposable elements extends lifespan in Caenorhabditis elegans. Nat Commun 14, 5278 (2023). https://doi.org/10.1038/s41467-023-40957-9